By Nathan Ohiomokhare
Health supply chain experts have been reviewing the federal governments nCovid 19 Vaccine distribution and administration strategy across Nigeria.
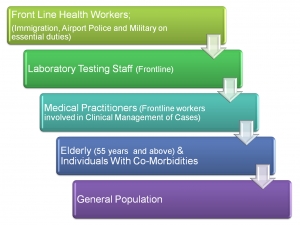
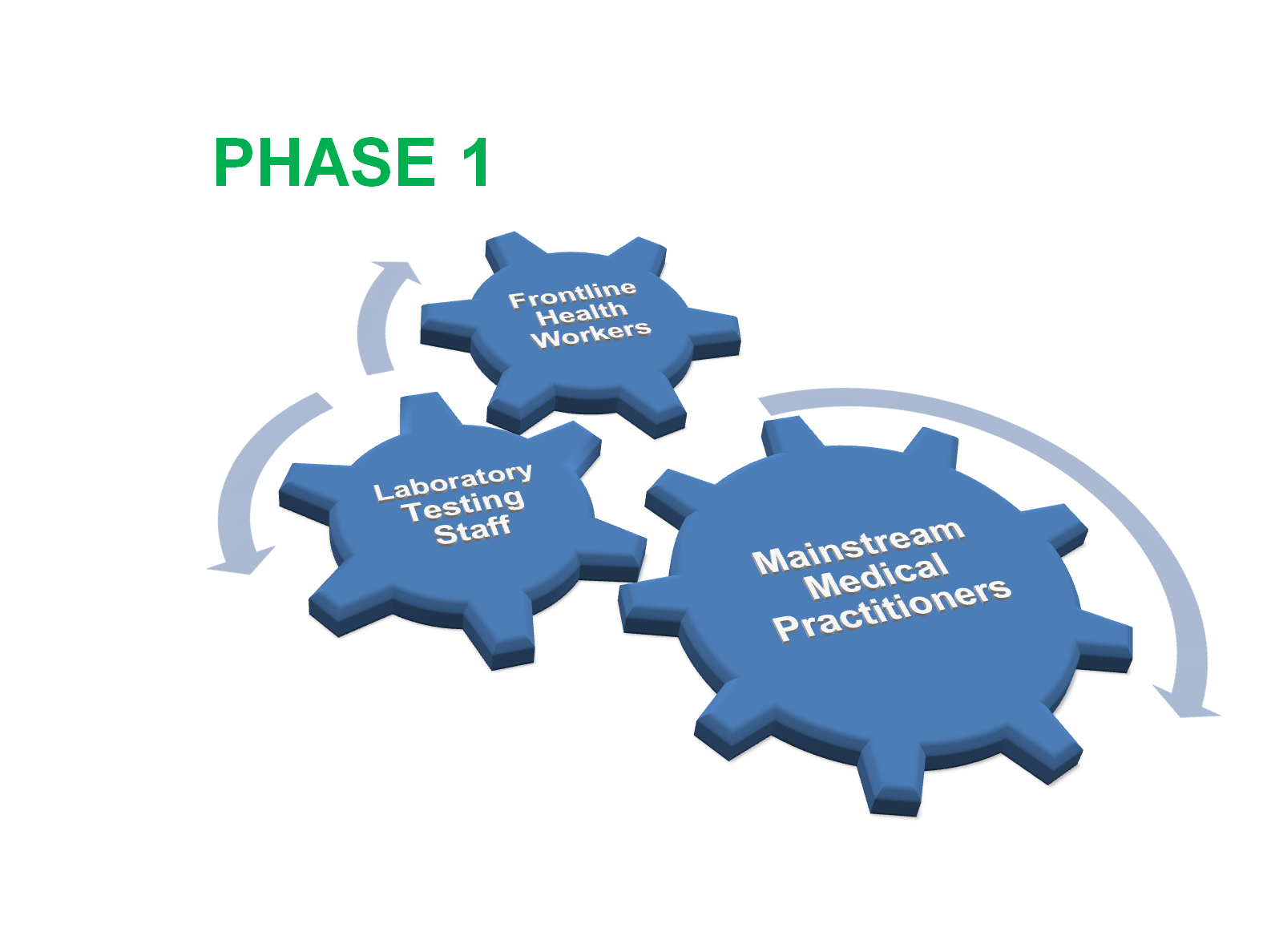
This month the National Primary Health Care Development Agency (NPHCDA) during an online sensitization seminar (webinar) released details of how batches of the nCOVID-19 vaccine doses will be distributed across Nigeria. According to the Executive Director Faisal Shuaib, NPHCDA will receive 100,000 doses of the novel Pfizer/BioNTech vaccine within the month of January 2021 with direct administration starting early February 2021. The strategy of the NPHCDA is to administer the Vaccine in 4 phases, targeting states with data showing higher percentage of patients positive for the nCov19 Virus. This would be after vaccinating frontline health workers, the elderly and vulnerable persons with co-morbidities based on World Health Organisation (WHO) guidelines. The aim is to achieve an appreciable level of herd immunity in these states with at least 40% of the entire population vaccinated by end of 2021 and additional 30% by in 2022.
As a precondition, the NPHCDA requires that cold chain storage facilities providing optimum conditions for storage and distribution of the temperature sensitive vaccines are put in place by states before receiving the vaccine.
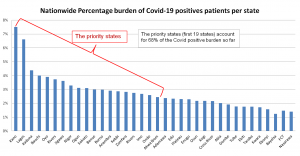
So the priority states are: Kano, Lagos, Katsina, Kaduna, Bauchi and Oyo will receive more doses for health workers.
The breakdown is as follows: Kano, 3,557; Lagos, 3,131; Katsina, 2,361; Kaduna, 2,074; Bauchi, 1,900; Oyo, 1,848; Rivers, 1,766; Jigawa, 1,712; Niger, 1,558; Ogun, 1,473; Sokoto, 1,468; Benue, 1,423; Borno, 1,416; Anambra, 1,379; Kebbi, 1,361; Zamfara, 1,336; Rivers, 1,306; Imo, 1,267; Ondo, 1,228; Akwa Ibom, 1,161.
Others are: Adamawa, 1,129; Edo, 1,104; Plateau, 1,089; Enugu, 1,088; Osun, 1,032; Kogi, 1,030; Cross River, 1,023; Abia, 955; Gombe, 908; Yobe, 842; Ekiti, 830; Taraba, 830; Kwara, 815; Ebonyi, 747; Bayelsa, 589; FCT, 695; Nasarawa, 661.
Requirements:
1) Cold chain storage facilities (warehousing and Transportation/ logistics)
2) Adequate Staffing to ensure administration of the doses within 5 days of receipt.
3) Data collection tools to ensure proper tracking, documentation and follow up.
4) Adequate mechanisms to ensure 100,000 doses to be administered to 50,000 people with 21 days. (Each individual is to take a second dose after 3 weeks)
Experts say implementation will require a seamless flow of information and agile decision-making structures between the Federal, state and Local governments with civil society and traditional organisations involved to ensure optimal mobilization of the population. The information Technology (IT) support has to be robust accurate. The existing technology infrastructure for previous vaccines programs will have to be leveraged and upgraded. Adequately trained staff and step down of training from Federal to state to LGA level and the rural communities is also needed. All this will require massive planning and orientation across the states.
The private sector healthcare institutions and logistics systems have to be brought onboard and partnered for shared learning because they are well exposed and very robust in their operations especially in the areas of Cold chain end-to-end supply chain management.
In all, experts agree it will be a continuous learning and improvement process requiring commitment from everyone involved.
Don't forget to drop a comment below

Good one from NPHCDA.
However as they partner the private sector healthcare facilities, they should ensure that licensed Clinical Community Pharmacists play a major role in the testing or screening, prescription and administration of the vaccines.
This makes it more accessible as Community pharmacies are the most accessible and visited healthcare facilities in any Community worldwide